To date, nothing is known about the molecular and cellular processes that these intranuclear bacteria use to infect and reproduce in animal hosts. A group of scientists from the Max Planck Institute for Marine Microbiology in Bremen, Germany, now presents the first in-depth analysis of an intranuclear parasite of animals in a study published in Nature Microbiology.
How to Massively Reproduce Within a Cell Without Killing It
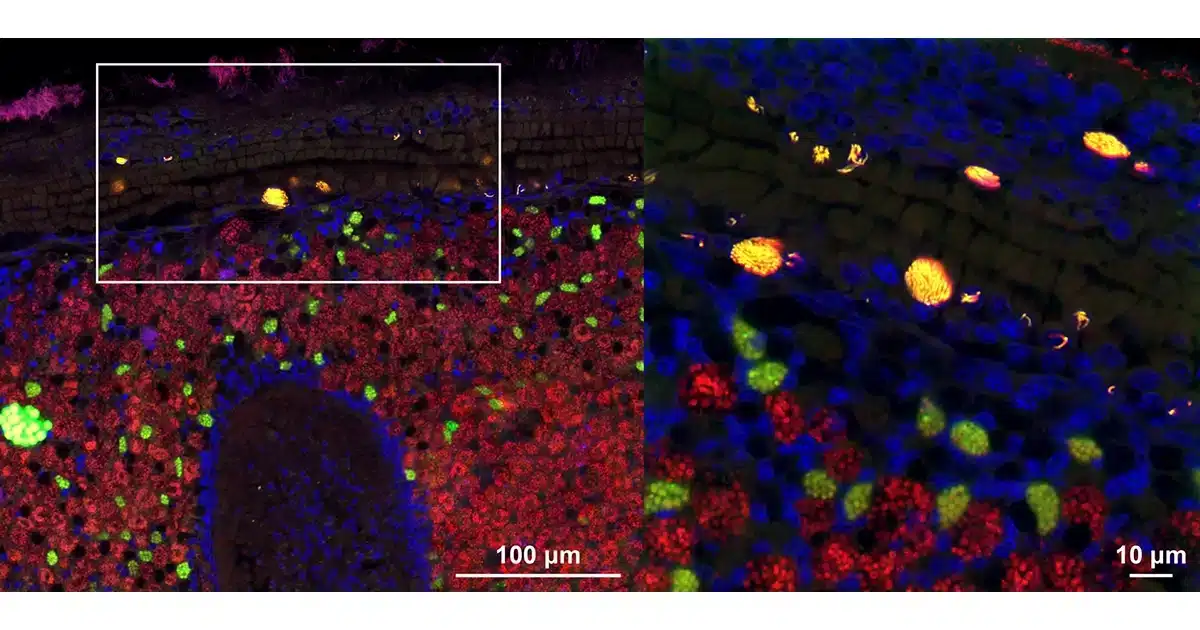
This intranuclear parasite, Candidatus Endonucleobacter, infects the nuclei of deep-sea mussels from hydrothermal vents and cold seeps around the world. A single bacterial cell penetrates into the mussels’ nucleus and then reproduces to over 80,000 cells, causing the nucleus to swell to 50 times its original size. “We wanted to understand how the bacterium infects and reproduces inside nuclei, and in particular how these bacteria acquire the nutrients they need for their massive replication, yet keep their host cells from dying,“ says Niko Leisch, co-senior author together with Nicole Dubilier from the Symbiosis Department at the Max Planck Institute for Marine Microbiology.
Using a suite of molecular and imaging methods, the scientists revealed that Ca. Endonucleobacter lives on sugars, lipids and other cell components from its host. It does not digest its host nucleic acids, like many other intranuclear bacteria. This feeding strategy ensures that the host cell functions long enough to provide Ca. Endonucleobacter with the nutrients it needs to reproduce to such massive numbers.
Arms Race for the Control of the Cell
A common response of animal cells to infection is apoptosis—a suicide program that cells initiate when they are damaged or infected by bacteria or viruses. “Interestingly, these bacteria have come up with a sophisticated strategy to keep their host cells from killing themselves,” says first author Miguel Ángel González Porras. “They produce proteins that suppress apoptosis called inhibitors of apoptosis (IAPs).” An arms race for the control of cell death then ensues: As the bacteria produce more and more IAPs, the host cell ramps up its production of proteins that induce apoptosis. Eventually, after the parasite has had enough time to multiply in masses, the host cell ruptures, releasing the bacteria and allowing them to infect new host cells.
Nicole Dubilier adds: “The discovery of IAPs in Ca. Endonucleobacter was one of the most surprising results of our study, because these proteins are only known from animals and a few viruses, but have never been found in bacteria.” The authors’ analyses of the evolutionary relationships of the IAPs revealed that the parasite likely acquired these genes from its host through horizontal gene transfer (HGT). While HGT from bacteria to eukaryotes is well known, only very few examples of HGT in the opposite direction—as the authors now found—are known.
Implications from Evolution to Medicine
“Our discovery expands our understanding of host-microbe interactions and highlights the complex strategies parasites have evolved to thrive in their hosts”, explains Nicole Dubilier. These findings could have broader implications for studying parasitic infections and immune evasion strategies in other organisms. “Our research sheds light on an overlooked mechanism of genetic exchange—HGT from eukaryotes to bacteria—potentially influencing how we understand microbial evolution and pathogenesis. Moreover, our study offers insights into apoptosis regulation, which is relevant to cancer research and cell biology,” Niko Leisch concludes.
Original Publication
Porras, M.Á.G., Assié, A., Tietjen, M. et al. An intranuclear bacterial parasite of deep-sea mussels expresses apoptosis inhibitors acquired from its host. Nat Microbiol (2024).https://doi.org/10.1038/s41564-024-01808-5